2025-07-29 06:35:37
Page Created majorPage Created June 1, 2025
Did you know?
Antibiotics can increase the risk of VVC. Antibiotics can disrupt the balance of the vaginal microbiome by reducing Lactobacillus levels, allowing Candida to overgrow and cause infection. This is why VVC is often a side effect of antibiotic treatment.
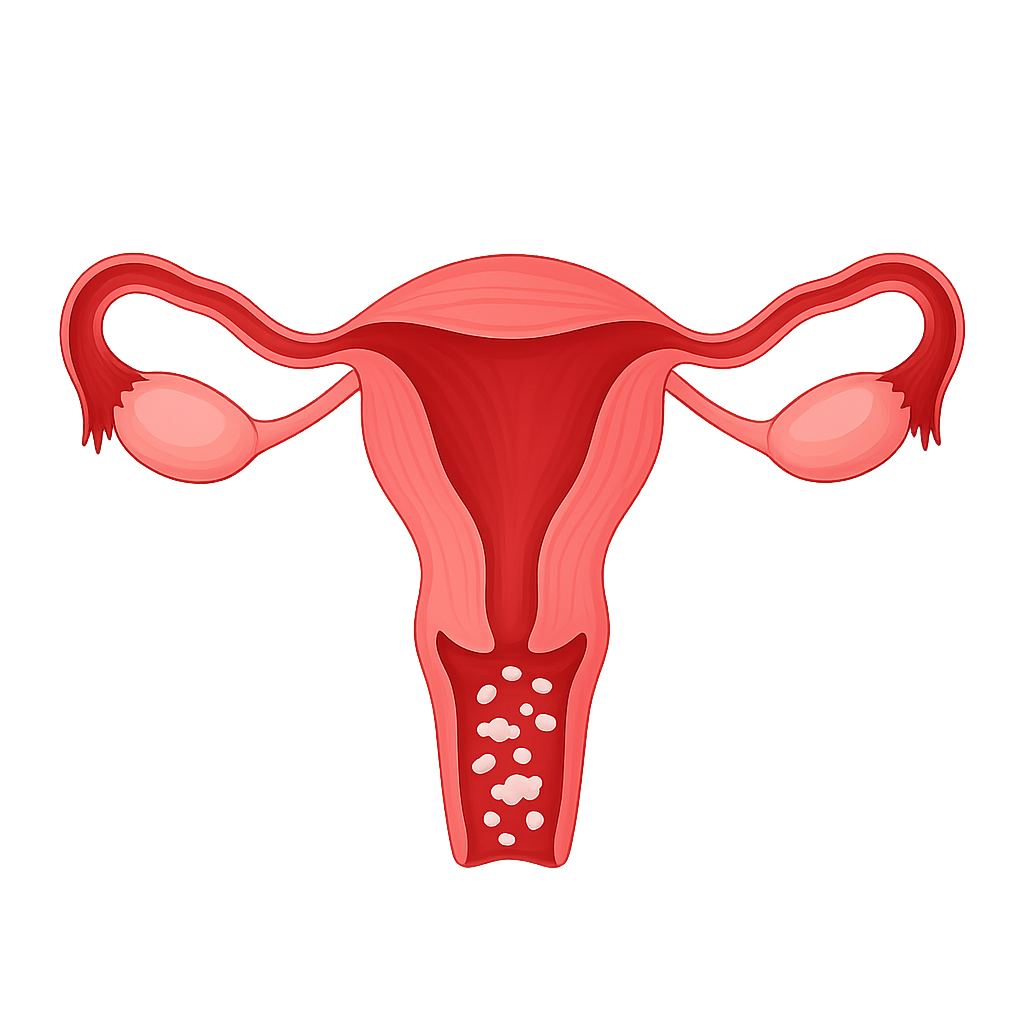
Vulvovaginal candidiasis (VC) is a common fungal infection caused by Candida albicans. Disruptions in the vaginal microbiome and immune responses contribute to its development. Effective treatment involves both antifungal therapy and strategies to restore microbiome balance, preventing recurrent infections and addressing emerging antifungal resistance.
I am a biochemist with a deep curiosity for the human microbiome and how it shapes human health, and I enjoy making microbiome science more accessible through research and writing. With 2 years experience in microbiome research, I have curated microbiome studies, analyzed microbial signatures, and now focus on interventions as a Microbiome Signatures and Interventions Research Coordinator.
Microbiome Signatures identifies and validates condition-specific microbiome shifts and interventions to accelerate clinical translation. Our multidisciplinary team supports clinicians, researchers, and innovators in turning microbiome science into actionable medicine.
I am a biochemist with a deep curiosity for the human microbiome and how it shapes human health, and I enjoy making microbiome science more accessible through research and writing. With 2 years experience in microbiome research, I have curated microbiome studies, analyzed microbial signatures, and now focus on interventions as a Microbiome Signatures and Interventions Research Coordinator.
Vulvovaginal candidiasis (VVC) is a highly prevalent fungal infection predominantly caused by Candida albicans and occasionally by non-albicans species such as C. glabrata and C. krusei. It affects approximately 70-75% of women at least once in their lifetime, with 5-8% experiencing recurrent vulvovaginal candidosis (RVVC), defined as four or more symptomatic episodes per year.[1] The disease manifests as an acute inflammatory condition of the vulva and vaginal mucosa, characterized by itching, burning, redness, and abnormal vaginal discharge. The pathogenesis is multifactorial, involving complex interactions among the Candida species’ virulence factors, host immune responses, estrogenic environment, and vaginal microbiome, primarily Lactobacilli.[2] Candida’s ability to switch between yeast and hyphal forms, form biofilms, and secrete toxins such as candidalysin contributes to infection persistence and symptom severity. Diagnosis remains challenging due to the presence of Candida as a commensal in many asymptomatic women.[3] Treatment mainly relies on azole antifungals, though increasing azole resistance, especially among non-albicans species, complicates management.[4][5] Long-term maintenance antifungal regimens are often necessary for RVVC but are associated with risks of resistance and relapse. Patient quality of life is significantly affected due to physical discomfort, psychological burden, and impaired sexual health. Advancing diagnostic accuracy, understanding microbiome-host interactions, and developing novel therapeutics are critical needs for improving clinical outcomes.
RVVC is linked with several associated conditions that modulate susceptibility and severity. High estrogen states such as pregnancy, use of oral contraceptives, and hormone replacement therapy increase the risk by altering vaginal immunity and microbiome balance.[6] Diabetes mellitus and immunosuppressive states, including HIV infection, also predispose women to frequent recurrences.[7][8] Antibiotic usage disrupts the protective Lactobacillus-dominant vaginal microbiota, facilitating Candida overgrowth.[9] Behavioral factors such as frequent sexual intercourse, use of poorly ventilated clothing, and atopic diseases may contribute to susceptibility. Genetic polymorphisms affecting innate immune signaling pathways have been implicated in impaired fungal clearance and enhanced inflammation. These factors, individually or in combination, create a permissive environment for Candida pathogenicity and chronic infection.
The etiology of VVC remains incompletely understood and is recognized as multifactorial. The primary causal theory revolves around an imbalance between Candida virulence mechanisms and host immune defenses. Candida albicans employs a polymorphic lifestyle, switching from benign yeast to invasive hyphal forms, secreting enzymes and candidalysin that promote tissue invasion and inflammation. Biofilm formation further shields Candida from antifungal agents.[10] Host factors impair fungal clearance, including estrogen-driven changes, neutrophil dysfunction, and genetic variants in pattern recognition receptors. The vaginal microbiome, particularly Lactobacilli, usually suppresses Candida growth; disruptions to this ecosystem via antibiotics or other factors remove this inhibition.[11] Limitations in current causal theories include insufficient understanding of the triggers converting asymptomatic colonization into symptomatic infection and the role of non-cultivable fungal species. Novel hypotheses suggest that immunological hypersensitivity to Candida antigens may drive symptoms independent of fungal load, highlighting the complexity of RVVC pathogenesis.[12]
| Theory | Description |
|---|---|
| Candida Virulence & Morphogenesis | Candida’s morphological switching and virulence factor secretion lead to tissue damage |
| Host Immune Dysfunction | Genetic and functional immune defects impair fungal clearance |
| Microbiome Dysbiosis | Disruption of Lactobacillus-dominated vaginal flora facilitates Candida overgrowth |
| Hypersensitivity Response | Exaggerated inflammatory reaction to Candida antigens causes symptoms |
Accurate diagnosis of VVC requires confirmation of Candida presence combined with clinical symptoms. Conventional diagnosis relies on clinical examination, microscopy to detect pseudohyphae or blastospores, and culture to identify Candida species.[13] However, microscopy sensitivity ranges from 50-80%, and culture may be slow or inconclusive. Molecular methods such as PCR enhance detection sensitivity and can identify mixed or resistant strains, but are not widely accessible.[14] Identification of Candida species is crucial, as non-albicans species often exhibit reduced susceptibility to azoles. Antifungal susceptibility testing is recommended in refractory cases.[15] Emerging research into microbiome and metabolomic signatures holds promise for non-invasive diagnostics that distinguish colonization from infection and identify treatment-resistant profiles.[16] Nonetheless, lack of point-of-care rapid testing and inconsistent guideline adherence contribute to delayed or inappropriate treatment, underscoring the need for improved diagnostics integrating microbiome insights.
VVC cannot be fully understood without considering its relationship with the vaginal microbiome, where beneficial bacteria such as Lactobacillus species maintain an acidic environment that suppresses fungal overgrowth. Beyond microbial interactions, the vaginal environment’s metal content plays a vital role. Essential metals like zinc and copper are tightly controlled by the host through nutritional immunity mechanisms to restrict fungal growth. Meanwhile, Candida has evolved strategies to acquire these metals despite host defenses. This dynamic interplay between the host’s immune system, resident microbes, and metal availability forms a complex network that shapes the course of VVC and offers new directions for diagnosis and treatment.
The metallomic signature of VVC reflects how Candida albicans interacts with and adapts to metal stress in the host environment. Research shows that C. albicans can tolerate and even sequester high concentrations of toxic metals like chromium, lead, and zinc, using biosorption mechanisms involving its cell wall.[17][x] These adaptations not only enhance its survival in hostile environments but may also reinforce its biofilm structure and resistance to antifungal agents. Additionally, differential sensitivity to metals like cadmium and mercury indicates the presence of complex detoxification and efflux pathways, which may overlap with drug resistance mechanisms. From a therapeutic perspective, disrupting Candida’s metal homeostasis, or exploiting its biosorptive capacity, could offer novel treatment strategies, particularly in drug-resistant or recurrent VVC cases. This metallomic insight expands our understanding of the infection beyond microbiome shifts, adding a crucial chemical-ecological layer to host-pathogen interactions.
Zinc (Zn
Zinc is a vital micronutrient that Candida albicans requires for enzymatic functions and virulence.[18] The host restricts zinc availability through sequestration proteins like calprotectin, which bind zinc to limit fungal growth.[19] In response, C. albicans expresses zinc scavenging proteins such as PRA1 and the high-affinity transporter ZRT1 to overcome this metal limitation.[20]
Copper (Cu)
Copper serves dual roles as an essential cofactor and a potential toxin. The host limits copper access to Candida by sequestration, forcing the fungus to adapt by downregulating copper-dependent enzymes like SOD1 and upregulating manganese-dependent alternatives such as SOD3.[21] Copper also exhibits antimicrobial properties that disrupt fungal respiration and ATP production.[22]
Lead (Pb)
Lead is a toxic heavy metal to which Candida albicans shows biosorption ability.[23][x] Although not essential for fungal growth, lead exposure influences fungal physiology and may affect biofilm formation. The full role of lead in VVC pathogenesis requires further study.
Mercury (Hg) and Silver (Ag)
Both mercury and silver possess potent antifungal effects by inhibiting cellular respiration and enzymatic activity in Candida.[24][25][x] Silver ions, in particular, demonstrate significant antifungal potential and are explored as topical treatments for resistant infections. Candida’s resistance to these metals is limited, highlighting their promise as adjunctive therapies.
Nutritional immunity is a key host defense mechanism in VVC, where the body restricts access to essential metals like zinc and copper to limit the growth and virulence of Candida albicans. During infection, immune cells such as neutrophils release calprotectin, a protein that tightly binds these metals, effectively starving the fungus of nutrients it needs to survive.[26] In response, C. albicans activates specialized metal-scavenging systems, including the zinc-binding protein PRA1 and transporter ZRT1, and shifts its antioxidant defenses to rely on manganese when copper is unavailable.[27] This ongoing struggle between host and pathogen, essentially a biochemical tug-of-war over micronutrients, plays a critical role in infection severity and persistence. When the vaginal microbiota, particularly protective Lactobacillus species, is disrupted, this defense can weaken, giving Candida a competitive edge.[28] Understanding nutritional immunity not only sheds light on the pathogenesis of VVC but also suggests new therapeutic directions, including strategies that enhance host metal-binding defenses or target fungal nutrient acquisition systems.
Our validation method evaluates the efficacy of microbiome-targeted interventions for VVC by correlating their influence on vaginal microbial balance, clinical symptom resolution, and the modulation of pathogenic mechanisms such as Candida overgrowth and biofilm formation. This approach distinguishes interventions that are validated, promising, or experimental based on the strength of evidence connecting changes in the vaginal microbiome with clinical improvement and relevant microbiological signatures.
| Intervention | Classification | Mechanism of Action | MBTI Status |
|---|---|---|---|
| Probiotics | Supplement | Replenish beneficial vaginal microbiota, competitively inhibit Candida, acidify vaginal pH, produce antifungal compounds and immunomodulate host response.[29][30] | Validated |
| Postbiotics | Supplement | Bioactive compounds released by probiotics, such as bacteriocins, SCFAs, and enzymes that inhibit pathogen growth and modulate inflammation.[31][x] | Promising Candidate |
| Prebiotics/Synbiotics | Supplement | Selectively enhance growth of beneficial microbes, thus indirectly suppressing Candida overgrowth; synbiotics combine prebiotics and probiotics.[32][33][x] | Promising Candidate |
| Vaginal Microbiome Transplantation (VMT) | Microbiota-based therapy | Transplant healthy donor vaginal microbiota to restore eubiosis and Lactobacillus dominance, reversing dysbiosis linked to VVC.[34][x] | Promising Candidate |
| Short chain fatty acid supplements | Supplement | Modulate vaginal pH, inhibit Candida growth and virulence, support mucosal immunity.[35][x][36] | Under investigation |
| Lactoferrin | Supplement | Chelates iron and other metals, impairs Candida growth, modulates inflammation, supports mucosal immunity.[37][38] | Promising candidate |
| Dietary Supplementation to maintain optimal calprotectin responses | Diet | Calciprotein binds and withholds essential metals (Zn, Cu), induces nutritional immunity, inhibits Candida growth and virulence gene expression.[39] | Experimental |
| Boric acid | Pharmaceutical | Disrupts Candida cell wall integrity, inhibits biofilm formation, restores vaginal acidity, effective against non-albicans and azole-resistant Candida species.[40] | Validated |
The vaginal microbiome is crucial in determining whether a woman will experience recurrent vulvovaginal candidiasis (RVVC). A healthy microbiome, dominated by Lactobacillus species, produces lactic acid, which helps maintain an acidic vaginal pH of around 4.5, an environment that inhibits Candida overgrowth. In women with RVVC, the vaginal microbiome often shows a reduced abundance of Lactobacillus species, with an overgrowth of other bacteria or fungi, allowing Candida to thrive. Additionally, factors like immune system dysfunction, such as neutrophil dysfunction, can impair the body’s ability to clear Candida effectively. Disruption of the microbiome can also lead to biofilm formation by Candida, which further shields the pathogen from antifungal treatments and immune responses, leading to persistent or recurrent infections. This imbalance in the vaginal microbiome plays a pivotal role in the recurrence of VVC, making its restoration through probiotics and other therapies a promising strategy for preventing RVVC.
Recurrent vulvovaginal candidiasis (RVVC) occurs in women who experience multiple episodes of VVC, typically four or more within a year. The key difference between women with RVVC and those with a single episode is often the underlying imbalance in the vaginal microbiome and the immune system. Women with RVVC commonly have a decreased abundance of Lactobacillus species, which leads to a higher pH environment and greater availability of nutrients for Candida growth. Additionally, women with RVVC may have an impaired immune response, with neutrophil dysfunction or genetic predispositions that prevent effective clearance of the yeast. The presence of biofilms, where Candida adheres to vaginal tissues and forms a protective matrix, is another factor contributing to recurrence. Biofilms make Candida more resistant to antifungal treatments and immune responses, leading to chronic infection. Hormonal changes, diabetes, antibiotic use, and stress can also act as triggers for recurrence, making RVVC more common in susceptible individuals.
Restoring a healthy vaginal microbiome is a crucial strategy for treating and preventing vulvovaginal candidiasis (VVC), especially in cases of recurrent infections. Probiotics containing Lactobacillus species are the most common intervention to help restore the balance of the vaginal microbiome. These probiotics work by outcompeting Candida for nutrients and space, as well as producing lactic acid to maintain the acidic pH that inhibits Candida growth. Probiotic treatments, whether oral or topical, have been shown to reduce the recurrence of VVC, particularly when used in conjunction with antifungal therapy. Furthermore, dietary changes that promote the growth of beneficial bacteria, such as consuming fiber-rich foods and limiting refined sugars, can support a healthier microbiome. In some cases, personalized microbiome testing may provide insights into specific imbalances, allowing for more targeted treatments, such as custom probiotic formulations, to restore a protective microbiota. As we learn more about the interactions between the microbiome, immune system, and Candida, future therapies may include even more precise approaches to manipulating the vaginal microbiome to prevent and treat VVC.
Did you know?
Antibiotics can increase the risk of VVC. Antibiotics can disrupt the balance of the vaginal microbiome by reducing Lactobacillus levels, allowing Candida to overgrow and cause infection. This is why VVC is often a side effect of antibiotic treatment.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Did you know?
Antibiotics can increase the risk of VVC. Antibiotics can disrupt the balance of the vaginal microbiome by reducing Lactobacillus levels, allowing Candida to overgrow and cause infection. This is why VVC is often a side effect of antibiotic treatment.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Did you know?
Antibiotics can increase the risk of VVC. Antibiotics can disrupt the balance of the vaginal microbiome by reducing Lactobacillus levels, allowing Candida to overgrow and cause infection. This is why VVC is often a side effect of antibiotic treatment.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Did you know?
Bacterial vaginosis (BV) increases the risk of acquiring HIV by up to 60% in women due to the disruption of the protective vaginal microbiome and the resulting inflammation that facilitates the virus’s entry.
Did you know?
Antibiotics can increase the risk of VVC. Antibiotics can disrupt the balance of the vaginal microbiome by reducing Lactobacillus levels, allowing Candida to overgrow and cause infection. This is why VVC is often a side effect of antibiotic treatment.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Did you know?
Antibiotics can increase the risk of VVC. Antibiotics can disrupt the balance of the vaginal microbiome by reducing Lactobacillus levels, allowing Candida to overgrow and cause infection. This is why VVC is often a side effect of antibiotic treatment.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Did you know?
Antibiotics can increase the risk of VVC. Antibiotics can disrupt the balance of the vaginal microbiome by reducing Lactobacillus levels, allowing Candida to overgrow and cause infection. This is why VVC is often a side effect of antibiotic treatment.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Did you know?
Antibiotics can increase the risk of VVC. Antibiotics can disrupt the balance of the vaginal microbiome by reducing Lactobacillus levels, allowing Candida to overgrow and cause infection. This is why VVC is often a side effect of antibiotic treatment.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
Did you know?
Bacterial vaginosis (BV) increases the risk of acquiring HIV by up to 60% in women due to the disruption of the protective vaginal microbiome and the resulting inflammation that facilitates the virus’s entry.
Alias iure reprehenderit aut accusantium. Molestiae dolore suscipit. Necessitatibus eum quaerat. Repudiandae suscipit quo necessitatibus. Voluptatibus ullam nulla temporibus nobis. Atque eaque sed totam est assumenda. Porro modi soluta consequuntur veritatis excepturi minus delectus reprehenderit est. Eveniet labore ut quas minima aliquid quibusdam. Vitae possimus fuga praesentium eveniet debitis exercitationem deleniti.
2025-07-29 06:35:37
Page Created majorPage Created June 1, 2025
2025-05-29 11:26:44
Vulvovaginal Candidiasis (VVC) majorpublished
Nutritional immunity restricts metal access to pathogens, leveraging sequestration, transport, and toxicity to control infections and immunity.
Microbiome Targeted Interventions (MBTIs) are cutting-edge treatments that utilize information from Microbiome Signatures to modulate the microbiome, revolutionizing medicine with unparalleled precision and impact.
Boric acid restores microbial balance in the vagina by increasing Lactobacillus and reducing harmful species, making it an effective treatment for recurrent BV and VVC.
Women’s health, a vital aspect of medical science, encompasses various conditions unique to women’s physiological makeup. Historically, women were often excluded from clinical research, leading to a gap in understanding the intricacies of women’s health needs. However, recent advancements have highlighted the significant role that the microbiome plays in these conditions, offering new insights and potential therapies. MicrobiomeSignatures.com is at the forefront of exploring the microbiome signature of each of these conditions to unravel the etiology of these diseases and develop targeted microbiome therapies.
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by Candida albicans. Disruptions in the vaginal microbiome and immune responses contribute to its development. Effective treatment involves both antifungal therapy and strategies to restore microbiome balance, preventing recurrent infections and addressing emerging antifungal resistance.
Women’s health, a vital aspect of medical science, encompasses various conditions unique to women’s physiological makeup. Historically, women were often excluded from clinical research, leading to a gap in understanding the intricacies of women’s health needs. However, recent advancements have highlighted the significant role that the microbiome plays in these conditions, offering new insights and potential therapies. MicrobiomeSignatures.com is at the forefront of exploring the microbiome signature of each of these conditions to unravel the etiology of these diseases and develop targeted microbiome therapies.
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by Candida albicans. Disruptions in the vaginal microbiome and immune responses contribute to its development. Effective treatment involves both antifungal therapy and strategies to restore microbiome balance, preventing recurrent infections and addressing emerging antifungal resistance.
Women’s health, a vital aspect of medical science, encompasses various conditions unique to women’s physiological makeup. Historically, women were often excluded from clinical research, leading to a gap in understanding the intricacies of women’s health needs. However, recent advancements have highlighted the significant role that the microbiome plays in these conditions, offering new insights and potential therapies. MicrobiomeSignatures.com is at the forefront of exploring the microbiome signature of each of these conditions to unravel the etiology of these diseases and develop targeted microbiome therapies.
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by Candida albicans. Disruptions in the vaginal microbiome and immune responses contribute to its development. Effective treatment involves both antifungal therapy and strategies to restore microbiome balance, preventing recurrent infections and addressing emerging antifungal resistance.
Women’s health, a vital aspect of medical science, encompasses various conditions unique to women’s physiological makeup. Historically, women were often excluded from clinical research, leading to a gap in understanding the intricacies of women’s health needs. However, recent advancements have highlighted the significant role that the microbiome plays in these conditions, offering new insights and potential therapies. MicrobiomeSignatures.com is at the forefront of exploring the microbiome signature of each of these conditions to unravel the etiology of these diseases and develop targeted microbiome therapies.
Bacterial vaginosis (BV) is caused by an imbalance in the vaginal microbiota, where the typically dominant Lactobacillus species are significantly reduced, leading to an overgrowth of anaerobic and facultative bacteria.
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by Candida albicans. Disruptions in the vaginal microbiome and immune responses contribute to its development. Effective treatment involves both antifungal therapy and strategies to restore microbiome balance, preventing recurrent infections and addressing emerging antifungal resistance.
Women’s health, a vital aspect of medical science, encompasses various conditions unique to women’s physiological makeup. Historically, women were often excluded from clinical research, leading to a gap in understanding the intricacies of women’s health needs. However, recent advancements have highlighted the significant role that the microbiome plays in these conditions, offering new insights and potential therapies. MicrobiomeSignatures.com is at the forefront of exploring the microbiome signature of each of these conditions to unravel the etiology of these diseases and develop targeted microbiome therapies.
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by Candida albicans. Disruptions in the vaginal microbiome and immune responses contribute to its development. Effective treatment involves both antifungal therapy and strategies to restore microbiome balance, preventing recurrent infections and addressing emerging antifungal resistance.
Women’s health, a vital aspect of medical science, encompasses various conditions unique to women’s physiological makeup. Historically, women were often excluded from clinical research, leading to a gap in understanding the intricacies of women’s health needs. However, recent advancements have highlighted the significant role that the microbiome plays in these conditions, offering new insights and potential therapies. MicrobiomeSignatures.com is at the forefront of exploring the microbiome signature of each of these conditions to unravel the etiology of these diseases and develop targeted microbiome therapies.
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by Candida albicans. Disruptions in the vaginal microbiome and immune responses contribute to its development. Effective treatment involves both antifungal therapy and strategies to restore microbiome balance, preventing recurrent infections and addressing emerging antifungal resistance.
Women’s health, a vital aspect of medical science, encompasses various conditions unique to women’s physiological makeup. Historically, women were often excluded from clinical research, leading to a gap in understanding the intricacies of women’s health needs. However, recent advancements have highlighted the significant role that the microbiome plays in these conditions, offering new insights and potential therapies. MicrobiomeSignatures.com is at the forefront of exploring the microbiome signature of each of these conditions to unravel the etiology of these diseases and develop targeted microbiome therapies.
Vulvovaginal candidiasis (VVC) is a common fungal infection caused by Candida albicans. Disruptions in the vaginal microbiome and immune responses contribute to its development. Effective treatment involves both antifungal therapy and strategies to restore microbiome balance, preventing recurrent infections and addressing emerging antifungal resistance.
Women’s health, a vital aspect of medical science, encompasses various conditions unique to women’s physiological makeup. Historically, women were often excluded from clinical research, leading to a gap in understanding the intricacies of women’s health needs. However, recent advancements have highlighted the significant role that the microbiome plays in these conditions, offering new insights and potential therapies. MicrobiomeSignatures.com is at the forefront of exploring the microbiome signature of each of these conditions to unravel the etiology of these diseases and develop targeted microbiome therapies.
Bacterial vaginosis (BV) is caused by an imbalance in the vaginal microbiota, where the typically dominant Lactobacillus species are significantly reduced, leading to an overgrowth of anaerobic and facultative bacteria.
Sustr, V., Foessleitner, P., Kiss, H., & Farr, A. (2020)
Vulvovaginal Candidosis: Current Concepts, Challenges and PerspectivesJournal of Fungi, 6(4), 267
Read ReviewKalia, N., Singh, J. & Kaur, M.
Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: a critical reviewAnn Clin Microbiol Antimicrob 19, 5 (2020)
Read ReviewDonders, G., Sziller, I. O., Paavonen, J., Hay, P., De Seta, F., Bohbot, J. M., Kotarski, J., Vives, J. A., Szabo, B., Cepuliené, R., & Mendling, W. (2022)
Management of recurrent vulvovaginal candidosis: Narrative review of the literature and European expert panel opinionFrontiers in Cellular and Infection Microbiology, 12, 934353
Sustr, V., Foessleitner, P., Kiss, H., & Farr, A. (2020)
Vulvovaginal Candidosis: Current Concepts, Challenges and PerspectivesJournal of Fungi, 6(4), 267
Read ReviewDonders, G., Sziller, I. O., Paavonen, J., Hay, P., De Seta, F., Bohbot, J. M., Kotarski, J., Vives, J. A., Szabo, B., Cepuliené, R., & Mendling, W. (2022)
Management of recurrent vulvovaginal candidosis: Narrative review of the literature and European expert panel opinionFrontiers in Cellular and Infection Microbiology, 12, 934353
Kumwenda P, Cottier F, Hendry AC, Kneafsey D, Keevan B, Gallagher H, Tsai HJ, Hall RA.
Estrogen promotes innate immune evasion of Candida albicans through inactivation of the alternative complement systemCell Rep. 2022 Jan 4;38(1):110183
Rosati D, Bruno M, Jaeger M, Ten Oever J, Netea MG.
Recurrent Vulvovaginal Candidiasis: An Immunological PerspectiveMicroorganisms. 2020 Jan 21;8(2):144
Oliveira PM, Mascarenhas RE, Lacroix C, Ferrer SR, Oliveira RP, Cravo EA, Alves AP, Grassi MF.
Candida species isolated from the vaginal mucosa of HIV-infected women in Salvador, Bahia, BrazilBraz J Infect Dis. 2011 May-Jun;15(3):239-44
Li H, Miao MX, Jia CL, Cao YB, Yan TH, Jiang YY, Yang F.
Interactions between Candida albicans and the resident microbiotaFront Microbiol. 2022 Sep 20;13:930495. doi:
Donders, G., Sziller, I. O., Paavonen, J., Hay, P., De Seta, F., Bohbot, J. M., Kotarski, J., Vives, J. A., Szabo, B., Cepuliené, R., & Mendling, W. (2022)
Management of recurrent vulvovaginal candidosis: Narrative review of the literature and European expert panel opinionFrontiers in Cellular and Infection Microbiology, 12, 934353
Horng H-C, Xu J-W, Kuo Y-S, Chen Y-S, Chiu Y-H, Tsui K-H, Tung Y-T.
Dual Mechanisms of Action: Anti-Candida and Anti-Inflammatory Potential of Lactobacillus Fermentation Broth in Treating Vulvovaginal CandidiasisJournal of Fungi. 2025; 11(1):18
Read ReviewRosati D, Bruno M, Jaeger M, ten Oever J, Netea MG.
Recurrent Vulvovaginal Candidiasis: An Immunological PerspectiveMicroorganisms. 2020; 8(2):144.
Donders, G., Sziller, I. O., Paavonen, J., Hay, P., De Seta, F., Bohbot, J. M., Kotarski, J., Vives, J. A., Szabo, B., Cepuliené, R., & Mendling, W. (2022)
Management of recurrent vulvovaginal candidosis: Narrative review of the literature and European expert panel opinionFrontiers in Cellular and Infection Microbiology, 12, 934353
Fan W, Li J, Chen L, Wu W, Li X, Zhong W, Pan H.
Clinical Evaluation of Polymerase Chain Reaction Coupled with Quantum Dot Fluorescence Analysis for Diagnosis of Candida Infection in Vulvovaginal Candidiasis PracticeInfect Drug Resist. 2023 Jul 25;16:4857-4865
Kucukates E, Gultekin NN, Alisan Z, Hondur N, Ozturk R.
Identification of Candida species and susceptibility testing with Sensititre YeastOne microdilution panel to 9 antifungal agentsSaudi Med J. 2016 Jul;37(7):750-7
Zhao C,,Li Y, Chen B,Yue K,Su Z,Xu J, Xue W, Zhao G, Zhang L,,,2023.
Mycobiome Study Reveals Different Pathogens of Vulvovaginal Candidiasis Shape Characteristic Vaginal BacteriomeMicrobiol Spectr11:e03152-22
Rodríguez, I. A., Cárdenas-González, J. F., Juárez, V. M. M., Pérez, A. R., Zarate, M. de G. M., & Castillo, N. C. P. (2018)
Biosorption of Heavy Metals by Candida albicans.InTech.
Lopes JP, Lionakis MS.
Pathogenesis and virulence of Candida albicansVirulence. 2022 Dec;13(1):89-121
Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC.
Role of Calprotectin in Withholding Zinc and Copper from Candida albicansInfect Immun. 2018 Jan 22;86(2):e00779-17
Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC.
Role of Calprotectin in Withholding Zinc and Copper from Candida albicansInfect Immun. 2018 Jan 22;86(2):e00779-17
Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC.
Role of Calprotectin in Withholding Zinc and Copper from Candida albicansInfect Immun. 2018 Jan 22;86(2):e00779-17
Salah I, Parkin IP, Allan E.
Copper as an antimicrobial agent: recent advancesRSC Adv. 2021 May 19;11(30):18179-18186
Rodríguez, I. A., Cárdenas-González, J. F., Juárez, V. M. M., Pérez, A. R., Zarate, M. de G. M., & Castillo, N. C. P. (2018)
Biosorption of Heavy Metals by Candida albicans.InTech.
Ayanwale AP, Estrada-Capetillo BL, Reyes-López SY.
Evaluation of Antifungal Activity by Mixed Oxide Metallic Nanocomposite against Candida spp.Processes. 2021; 9(5):773
Górka K, Kubiński K.
Antifungal Activity against Human and Plant Mycopathogens, and Green Synthesis of Silver Nanoparticles Exhibiting Such ActivityApplied Sciences. 2024; 14(1):115.
Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC.
Role of Calprotectin in Withholding Zinc and Copper from Candida albicansInfect Immun. 2018 Jan 22;86(2):e00779-17
Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC.
Role of Calprotectin in Withholding Zinc and Copper from Candida albicansInfect Immun. 2018 Jan 22;86(2):e00779-17
Zangl I, Pap IJ, Aspöck C, Schüller C.
The role of Lactobacillus species in the control of Candida via biotrophic interactionsMicrob Cell. 2019 Nov 25;7(1):1-14
Zahedifard T, Khadivzadeh T, Rakhshkhorshid M.
The Role of Probiotics in the Treatment of Vulvovaginal Candidiasis: A Systematic Review and Meta-AnalysisEthiop J Health Sci. 2023 Sep;33(5):881-890
Abavisani, Mohammad, Saeed Sahebi, Farhad Dadgar, Farzaneh Peikfalak, and Masoud Keikha
The Role of Probiotics as Adjunct Treatment in the Prevention and Management of Gynecological Infections: An Updated Meta-analysis of 35 RCT StudiesTaiwanese Journal of Obstetrics and Gynecology 63, no. 3 (2024): 357-368. Accessed June 6, 2025.
Wang, Y., Liu, Z., & Chen, T. (2024).
Vaginal microbiota: Potential targets for vulvovaginal candidiasis infectionHeliyon, 10(5), e27239.
Read ReviewGandhi AB, Purandare A, Athota K, et al.
Prebiotics and Probiotics in Vulvovaginal InfectionsJ South Asian Feder Obst Gynae 2022;14(3):343–346.
Wang, Y., Liu, Z., & Chen, T. (2024).
Vaginal microbiota: Potential targets for vulvovaginal candidiasis infectionHeliyon, 10(5), e27239.
Read ReviewWang, Y., Liu, Z., & Chen, T. (2024).
Vaginal microbiota: Potential targets for vulvovaginal candidiasis infectionHeliyon, 10(5), e27239.
Read ReviewWang, Y., Liu, Z., & Chen, T. (2024).
Vaginal microbiota: Potential targets for vulvovaginal candidiasis infectionHeliyon, 10(5), e27239.
Read ReviewBaldewijns, Silke, Mart Sillen, Ilse Palmans, Paul Vandecruys, Patrick Van Dijck, and Liesbeth Demuyser.
The Role of Fatty Acid Metabolites in Vaginal Health and Disease: Application to CandidiasisFrontiers in Microbiology 12, (2021): 705779. Accessed June 6, 2025.
Costantino D, Guaraldi C. Studio preliminare sull'utilizzo di una crema contenente Lattoferrina nel trattamento della vulvovaginite acuta da candida
Preliminary evaluation of a vaginal cream containing lactoferrin in the treatment of vulvovaginal candidosisMinerva Ginecol. 2008 Apr;60(2):121-5. Italian.
Russo, Rosario, Fabiana Superti, Eugen Karadja, and Francesco D. Seta.
Randomised Clinical Trial in Women with Recurrent Vulvovaginal Candidiasis: Efficacy of Probiotics and Lactoferrin as Maintenance TreatmentMycoses 62, no. 4 (2019): 328-335. Accessed June 6, 2025.
Besold AN, Gilston BA, Radin JN, Ramsoomair C, Culbertson EM, Li CX, Cormack BP, Chazin WJ, Kehl-Fie TE, Culotta VC.
Role of Calprotectin in Withholding Zinc and Copper from Candida albicansInfect Immun. 2018 Jan 22;86(2):e00779-17
Powell A, Ghanem KG, Rogers L, Zinalabedini A, Brotman RM, Zenilman J, Tuddenham S.
Clinicians’ Use of Intravaginal Boric Acid Maintenance Therapy for Recurrent Vulvovaginal Candidiasis and Bacterial Vaginosis.Sexually Transmitted Diseases 46(12):p 810-812, December 2019.
Read Review