Did you know?
In chronic kidney disease, up to 70 percent of circulating uremic toxins come from gut bacteria rather than the failing kidneys themselves. Lactulose can selectively suppress the very bacterial groups that generate these toxins.
Lactulose: A Validated Microbiome-Targeted Intervention for CKD Management
Lactulose functions as a validated microbiome-targeted intervention for KD by suppressing dominant pathogenic taxa such as Shigella while enriching depleted CFA-producing bacteria like Bifidobacterium. This dual modulation reduces uremic toxin generation, restores intestinal function, and mechanistically slows KD progression through targeted correction of dysbiosis.
-
Karen Pendergrass
Read MoreKaren Pendergrass is a microbiome researcher specializing in microbiome-targeted interventions (MBTIs). She systematically analyzes scientific literature to identify microbial patterns, develop hypotheses, and validate interventions. As the founder of the Microbiome Signatures Database, she bridges microbiome research with clinical practice. In 2012, based on her own investigative research, she became the first documented case of FMT for Celiac Disease—four years before the first published case study.
Microbiome Signatures identifies and validates condition-specific microbiome shifts and interventions to accelerate clinical translation. Our multidisciplinary team supports clinicians, researchers, and innovators in turning microbiome science into actionable medicine.
Karen Pendergrass is a microbiome researcher specializing in microbiome-targeted interventions (MBTIs). She systematically analyzes scientific literature to identify microbial patterns, develop hypotheses, and validate interventions. As the founder of the Microbiome Signatures Database, she bridges microbiome research with clinical practice. In 2012, based on her own investigative research, she became the first documented case of FMT for Celiac Disease—four years before the first published case study.
Overview
Lactulose represents a validated microbiome-targeted intervention that operates through a dual mechanism: simultaneously decreasing pathogenic bacterial taxa while increasing short-chain fatty acid (SCFA)-producing bacteria that are characteristically depleted in chronic kidney disease (CKD).[1][2] This targeted approach addresses fundamental dysbiosis mechanisms underlying CKD progression and associated gastrointestinal complications.
Suppression of Pathogenic Bacterial Taxa Associated with CKD
A critical aspect of lactulose’s therapeutic efficacy involves the selective suppression of pathogenic bacterial genera that dominate dysbiotic states in CKD. Patients with acute pancreatitis—a condition sharing dysbiotic features with CKD—demonstrate that lactulose was superior to rhubarb in the modulation of gut microbiota, including the inhibition of Escherichia-Shigella and proliferation of Bifidobacterium, as well as the increased production of SCFAs.[3][4] More specifically, while Escherichia-Shigella and Enterococcus were diminished in the lactulose group, patients who received other interventions such as rhubarb instead harbored more abundant Escherichia-Shigella.[5] The specific reduction of Escherichia-Shigella through lactulose treatment is particularly significant because these gram-negative bacteria are recognized pathogenic species in dysbiosis states. In fact, dysbiosis is revealed by lower diversity and higher abundance of pathogenic bacteria, including Escherichia, Shigella, and Streptococcus.[6] The relative abundance of pathogenic bacteria such as Escherichia-Shigella was decreased after lactulose treatment in non-antibiotic groups, demonstrating lactulose’s selective antimicrobial effects on these dominant pathogenic taxa.[7]
Enrichment of SCFA-Producing Bacteria Depleted in CKD
Simultaneously with suppression of pathogenic bacteria, lactulose specifically enriches populations of beneficial SCFA-producing bacteria that are typically depleted in chronic kidney disease. The relative abundance of Bifidobacterium was significantly enriched in the gut of patients after lactulose administration, accompanied by higher concentrations of SCFAs.[8] In the CKD context specifically, the Bifidobacterium peak area ratio was increased more in lactulose-treated groups compared with control groups.[9] This enrichment is therapeutically crucial because Bifidobacterium and other beneficial bacteria are major SCFA producers, generating short-chain fatty acids through anaerobic fermentation of dietary fiber and resistant starch.[10] The restoration of these SCFA-producing communities addresses a fundamental depletion observed in CKD patients. Beyond simply enriching beneficial bacteria, lactulose could restore intestinal function while also regulating gut microbiota and promoting the production of SCFAs.[11] The specific SCFA metabolites produced—including acetic acid, propionic acid, and butyric acid—are restored to levels that support intestinal health and systemic metabolic function.
Mechanisms Underlying Pathogenic Taxa Reduction and SCFA Producer Enrichment
The mechanisms by which lactulose achieves this dual effect reflect its unique physicochemical properties. Lactulose reaches the large intestine in its unchanged form when administered orally because the human small intestine lacks the enzymes necessary to split it into its component monosaccharides.[12] Once in the colon, lactulose is selectively metabolized by resident colonic bacteria and subsequently produces large amounts of SCFAs. This selective fermentation by beneficial bacteria creates a prebiotic environment that selectively favors SCFA-producing bacteria while creating conditions unfavorable for pathogenic taxa. Additionally, lactulose functions as an osmotic laxative that increases osmotic pressure in the lower intestinal tract, helping to retain intestinal water and electrolytes, thereby stimulating bowel movements and improving intestinal motility.[13]
Clinical Evidence of CKD Progression Prevention
The validation of lactulose as a microbiome-targeted intervention is demonstrated through measurable clinical outcomes in CKD models. In adenine-induced CKD rat models, the 3.0% and 7.5% lactulose-containing diet groups improved serum creatinine (sCr) and blood urea nitrogen (BUN) levels, and suppressed tubulointerstitial fibrosis, suggesting prevention of CKD progression by lactulose.[14][15] Furthermore, lactulose altered the intestinal environment as a prebiotic and, thereby, suppressed uremic toxin production, consequently suppressing the renal disorder and tubulointerstitial fibrosis progression.[16][17]
Impact on Uremic Toxin-Producing Bacteria
The reduction of pathogenic bacteria specifically relates to their role in producing uremic toxins. In CKD, specific bacterial taxa including Bacteroides and Clostridium cluster XI—which contain many bacterial species producing indole, a precursor of the uremic toxin indoxyl sulfate (IS)—showed decreased peak area ratios in lactulose-treated groups compared with control groups.[18] This reduction of indole-producing bacteria directly translates to decreased serum IS levels, as both lactulose-treated groups showed significantly lower levels than the control group.[19] The decreased serum IS induced by lactulose was attributable to the suppressed metabolic conversion into indoles in the intestine, reflecting the reduced abundance of these bacterial taxa.
Broader Implications as a Validated Intervention
The comprehensive microbiota-targeted mechanism of lactulose—simultaneously depleting pathogenic taxa including Escherichia, Shigella, and Streptococcus groups while enriching SCFA-producing bacteria—establishes it as a validated intervention specifically designed to reverse the characteristic dysbiosis of CKD. This prebiotic lactulose represents a potent alternative intervention in the therapy of patients with gut failure, offering a mechanism-based approach to restoring microbial ecology and preventing CKD progression through microbiota modulation rather than purely symptomatic management.[20]
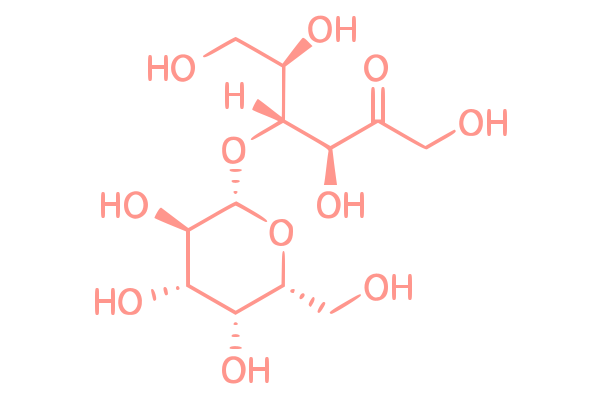
Lactulose is a non-absorbable disaccharide composed of galactose and fructose that functions as a prebiotic, a selectively fermented ingredient that promotes the growth of beneficial microorganisms in the gut.
Short-chain fatty acids are microbially derived metabolites that regulate epithelial integrity, immune signaling, and microbial ecology. Their production patterns and mechanistic roles provide essential functional markers within microbiome signatures and support the interpretation of MBTIs, MMAs, and systems-level microbial shifts across clinical conditions.
Dysbiosis in chronic kidney disease (CKD) reflects a shift toward reduced beneficial taxa and increased pathogenic, uremic toxin-producing species, driven by a bidirectional interaction in which the uremic environment disrupts microbial composition and dysbiotic metabolites accelerate renal deterioration.
Short-chain fatty acids are microbially derived metabolites that regulate epithelial integrity, immune signaling, and microbial ecology. Their production patterns and mechanistic roles provide essential functional markers within microbiome signatures and support the interpretation of MBTIs, MMAs, and systems-level microbial shifts across clinical conditions.
References
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial.. Tayebi-Khosroshahi H, Habibzadeh A, Niknafs B, Ghotaslou R, Yeganeh Sefidan F, Ghojazadeh M, et al.. (J Renal Inj Prev. 2016;5(3):162-167.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial.. Tayebi-Khosroshahi H, Habibzadeh A, Niknafs B, Ghotaslou R, Yeganeh Sefidan F, Ghojazadeh M, et al.. (J Renal Inj Prev. 2016;5(3):162-167.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.. Sueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.. (Clin Exp Nephrol. 2019;23:908-919.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.. Sueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.. (Clin Exp Nephrol. 2019;23:908-919.)
- Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.. Sueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.. (Clin Exp Nephrol. 2019;23:908-919.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.. Sueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.. (Clin Exp Nephrol. 2019;23:908-919.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
- Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.. Sueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.. (Clin Exp Nephrol. 2019;23:908-919.)
- Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.. Sueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.. (Clin Exp Nephrol. 2019;23:908-919.)
- Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.. Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.. (Biomed Pharmacother. 2023;163:114769.)
Wang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewTayebi-Khosroshahi H, Habibzadeh A, Niknafs B, Ghotaslou R, Yeganeh Sefidan F, Ghojazadeh M, et al.
The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial.J Renal Inj Prev. 2016;5(3):162-167.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewTayebi-Khosroshahi H, Habibzadeh A, Niknafs B, Ghotaslou R, Yeganeh Sefidan F, Ghojazadeh M, et al.
The effect of lactulose supplementation on fecal microflora of patients with chronic kidney disease; a randomized clinical trial.J Renal Inj Prev. 2016;5(3):162-167.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewSueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.
Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.Clin Exp Nephrol. 2019;23:908-919.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewSueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.
Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.Clin Exp Nephrol. 2019;23:908-919.
Read ReviewSueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.
Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.Clin Exp Nephrol. 2019;23:908-919.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewSueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.
Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.Clin Exp Nephrol. 2019;23:908-919.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read ReviewSueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.
Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.Clin Exp Nephrol. 2019;23:908-919.
Read ReviewSueyoshi M, Fukunaga M, Mei M, Nakajima A, Tanaka G, Murase T, et al.
Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats.Clin Exp Nephrol. 2019;23:908-919.
Read ReviewWang J, Jiang M, Hu Y, Lei Y, Zhu Y, Xiong H, He C.
Lactulose regulates gut microbiota dysbiosis and promotes short-chain fatty acids production in acute pancreatitis patients with intestinal dysfunction.Biomed Pharmacother. 2023;163:114769.
Read Review