Microbiome Signatures of Endometriosis: A 20-Year Literature Review
This literature review maps the microbiome signature of endometriosis across multiple body sites, revealing distinct taxa enriched or depleted in patients compared to controls, highlighting microbial shifts by disease stage, site, and taxa.
-
Karen Pendergrass
Karen Pendergrass is a microbiome researcher specializing in microbiome-targeted interventions (MBTIs). She systematically analyzes scientific literature to identify microbial patterns, develop hypotheses, and validate interventions. As the founder of the Microbiome Signatures Database, she bridges microbiome research with clinical practice. In 2012, based on her own investigative research, she became the first documented case of FMT for Celiac Disease—four years before the first published case study.
Microbiome Signatures identifies and validates condition-specific microbiome shifts and interventions to accelerate clinical translation. Our multidisciplinary team supports clinicians, researchers, and innovators in turning microbiome science into actionable medicine.
Karen Pendergrass is a microbiome researcher specializing in microbiome-targeted interventions (MBTIs). She systematically analyzes scientific literature to identify microbial patterns, develop hypotheses, and validate interventions. As the founder of the Microbiome Signatures Database, she bridges microbiome research with clinical practice. In 2012, based on her own investigative research, she became the first documented case of FMT for Celiac Disease—four years before the first published case study.
Overview
Endometriosis is a chronic inflammatory condition characterized by endometrial-like tissue outside the uterus. Growing evidence over the past two decades suggests that endometriosis is accompanied by microbiome disturbances (dysbiosis) in various body compartments. [1][2] Research has focused on comparing the microbiomes of endometriosis patients to healthy controls in niches including the vagina, gut, peritoneal fluid, endometrium (eutopic intrauterine tissue and ectopic lesions), the oral cavity, and even systemic circulation. Here we provide a comprehensive review of these studies, organizing findings by disease stage (early vs. advanced), microbial taxa (genus and species), and anatomical compartment. We highlight microbial taxa consistently reported as increased or decreased in endometriosis relative to controls, and summarize how these microbes may interact with metal ions (Ni, Zn, Cd, Pb, Fe) relevant to microbial ecology and endometriosis pathology. Tables and figures are included to summarize key comparative findings for clarity.
Key findings: Across compartments, endometriosis is often associated with subtle but distinct shifts in microbial composition compared to controls. For example, some studies report lower Lactobacillus and higher abundance of anaerobic or opportunistic bacteria in the vaginal microbiome of endometriosis patients. [3] In the gut, certain genera (e.g. Lachnospira, Oscillospira) have been found at higher levels in endometriosis, though results are not uniform. The peritoneal environment in endometriosis tends to harbor more Gram-negative and aerobic/facultative bacteria (like Pseudomonas and Streptococcus) and fewer commensal skin/oral microbes. The uterine (endometrial) microbiome of endometriosis patients can show an enrichment of potential pathogens such as Escherichia coli, Streptococcus, and Acinetobacter compared to controls. Notably, a Fusobacterium species – a known oral and intestinal anaerobe – has emerged as a recurrent finding in endometriosis lesions and oral samples of patients with moderate/severe disease. [4][5] We explore these findings in detail by body site below, then discuss the interplay between these microbes and metal ions (e.g. iron from menstrual blood fueling certain bacteria nickel as a cofactor for microbial enzymes which contributes to the endometriosis-microbiome relationship.
Vaginal Microbiome in Endometriosis
Composition in endometriosis vs. healthy controls: The vaginal microbiome in healthy women is typically dominated by lactobacilli, which maintain a low pH and inhibit pathogens. Endometriosis patients also generally retain Lactobacillus dominance in the vagina, but several studies report shifts in specific taxa. [x] [x] For example, Wei et al. (2020) found that while Lactobacillus remained the most abundant genus, women with endometriosis had enrichment of Aerococcus and Prevotella in the vaginal microbiome compared to controls. Similarly, a study by Chao et al. (2021) noted that endometriosis (with chronic pelvic pain) was associated with a lower relative abundance of Lactobacillus (especially L. jensenii) and higher abundance of anaerobes such as Clostridium (including C. butyricum), Alloscardovia, Veillonella, and Stenotrophomonas in vaginal samples. These changes suggest a shift from an optimal Lactobacillus-dominated community to a more diverse, dysbiotic profile in some patients.
Other investigations, however, have reported minimal differences. Perrotta et al. (2020) observed no significant compositional differences in vaginal microbiota between 35 endometriosis patients (mixed stages I–IV) and 24 controls. Hernandes et al. (2020) similarly found that Lactobacillus overwhelmingly predominated in vaginal fluid of both deep endometriosis patients and controls, and interestingly noted that Gardnerella and Prevotella were lower in endometriosis patients’ vaginal samples than in controls. This latter finding contrasts with the expectation of a “bacterial vaginosis (BV)-like” increase in anaerobes; it suggests that not all endometriosis patients exhibit vaginal dysbiosis, and some may even have fewer BV-associated bacteria than controls. The discrepancies across studies may stem from differences in cohorts (symptomatic vs. asymptomatic, ethnic background, etc.) and methodologies. Nonetheless, a theme emerges that when differences are present, endometriosis is often associated with higher levels of certain non-Lactobacillus anaerobes or facultative bacteria in the vagina. [6]
Disease stage influence
Advanced endometriosis might exacerbate vaginal microbiome changes. Perrotta et al. reported that during menses, women with stage III–IV disease had a significantly different vaginal microbial profile than those with stage I–II, characterized by enrichment of Anaerococcus (an obligate anaerobe) in the higher-stage group. Likewise, Wei et al. observed that Prevotella and Aerococcus increases were most pronounced in endometriosis patients (stages I–IV) compared to controls, and another study noted Megasphaera (an anaerobe) was decreased in endo with pain, with corresponding increases in Clostridium and Alloscardovia. [7] These data hint that severe endometriosis/pelvic pain states correlate with a less lactobacilli-centric vaginal microbiome (more diverse anaerobes). However, not all stage-related trends are intuitive; one study from China found that advanced endometriosis was actually associated with higher Lactobacillus (in the cervical microbiome) alongside increased Streptococcus and reduced Dialister, compared to milder disease. [8]This could reflect an increase in Lactobacillus iners (a Lactobacillus species often seen in disrupted vaginal communities) rather than the protective L. crispatus. Overall, stage III–IV endometriosis may predispose to greater microbiome perturbation in the lower genital tract, but findings are mixed.
Table 1 summarizes the taxa reported as increased or decreased in the vaginal microbiome of endometriosis patients across representative studies.
Table 1. Vaginal Microbiome Differences in Endometriosis vs. Controls (selected taxa that are consistently reported).
| Taxon | Change in Endometriosis | Notes/Study References |
|---|---|---|
| Lactobacillus spp. | ↓ (often) or no change | Often slightly lower in endometriosis, especially L. jensenii. Some studies show no significant change. [9] |
| Gardnerella | ↑ or ↓ (contradictory) | Enriched in some endo patients but decreased in deep endometriosis patients in one study. [10][11] |
| Prevotella | ↑ (common in dysbiosis) | Higher in some endo vaginas; associated with pain + endometriosis. [12] |
| Aerococcus | ↑ | Enriched in endo vaginal samples. [13] |
| Anaerococcus | ↑ (advanced stage) | Higher in stage III–IV endo (menstrual phase).[14] |
| Clostridium, Veillonella, Alloscardovia | ↑ (with pelvic pain) | Higher in endo + chronic pain group. [15] |
| Megasphaera, Shuttleworthia | ↓ (with pain) | Lower in endo + pain vs pain-only controls. [16] |
| Atopobium | ↓ (in some endo) | Absent in endo vaginal samples in one study. [17] |
(↑ = increased abundance in endometriosis; ↓ = decreased in endometriosis, relative to controls.)
Gut Microbiome (Stool) in Endometriosis
The gut microbiome has been intensively studied for its role in systemic inflammation and estrogen metabolism (via the “estrobolome”). Several small case–control studies have identified particular gut microbial differences in endometriosis, though a consistent signature is still being established. In a high-quality study by Svensson et al. (2021) with 66 endometriosis cases and 198 population controls, only three genera differed significantly: Lachnospira, Oscillospira, and an unclassified genus in order Bacteroidales were more abundant in endometriosis patients’ stool compared to controls. These genera are associated with fiber fermentation and butyrate production, and their increase in endometriosis could reflect subtle community shifts. By contrast, an earlier study by Ata et al. (2019) found that Sneathia, Barnesiella, and Gardnerella were significantly decreased in the fecal microbiota of endometriosis patients vs. controls (all P<0.001). [18] The drop in Sneathia and Gardnerella in stool might seem counterintuitive, as these are genera typically known from the genital tract; their presence in stool may indicate oral/vaginal to gut transfer or low-level gut colonization that is somehow reduced in endometriosis. It’s worth noting that Gardnerella was also reported to be lower in the vaginal and endometrial samples of those same patients, hinting that certain women without endometriosis in that study had more Gardnerella both vaginally and intestinally (perhaps due to undiagnosed BV or other factors). [19]
Large-scale data suggest that gut microbiome differences in endometriosis may be subtle. A recent cohort study on ~1000 individuals (136 endometriosis vs. 864 controls) found no significant overall differences in gut microbial diversity or composition between endometriosis patients and controls. Neither alpha-diversity nor beta-diversity showed clear separation, and no reproducible taxonomic biomarkers emerged in that large sample. The authors noted that previous smaller studies using 16S rRNA gene profiling reported some differences, but these often did not replicate at species-level resolution. This suggests that while gut dysbiosis might play a role in endometriosis pathogenesis for some individuals, there is not yet a universal gut microbiome signature of the disease. Differences may depend on diet, geography, or endometriosis phenotype. [20]
Nonetheless, a qualitative trend mentioned in several reports (including animal models) is an altered Firmicutes/Bacteroidetes ratio. For example, a mouse study by Yuan et al. (2018) found enrichment of Firmicutes and Actinobacteria and reduced Lactobacillus in the gut of mice with induced endometriosis. [21] Conversely, some human studies have noted increased Bacteroidetes in patients.[22] However, in the context of inflammation, a noteworthy observation is that members of the anti-inflammatory family Ruminococcaceae (butyrate producers) tend to be lower in inflammatory conditions and in endometriosis as well. For instance, an inverse correlation between Ruminococcus abundance and IL-6 levels has been described, aligning with the chronic inflammatory milieu of endometriosis. [23]
In summary, gut microbiome changes in endometriosis are inconsistently reported. Some patients show a dysbiosis pattern (e.g. lower beneficial commensals like Ruminococcaceae and higher pro-inflammatory taxa), but large studies indicate that many endometriosis patients have gut microbiomes within the range of healthy variation. It is possible that only a subset of endometriosis patients (perhaps those with co-morbid gut symptoms or higher toxic exposures) have pronounced gut dysbiosis. Further research, especially integrating metabolic readouts, is needed to clarify the gut microbiome’s role in endometriosis.[24]
Disease stage influence
There is limited evidence that the endometriosis stage strongly affects the gut microbiome composition. One small study on stress and endometriosis (Xu et al. 2017) did note that in patients with chronic stress, certain gut bacteria (Paraprevotella, Odoribacter, Veillonella, Ruminococcus) were further decreased and Prevotella increased, compared to non-stressed endo patients– implying stress plus endo might exacerbate dysbiosis. [25] However, in terms of stage (I/II vs. III/IV), the literature does not show a clear, reproducible difference in gut microbiota profiles. The large cohort study mentioned above did not find significant microbiome differences even when restricted to more severe cases, for example. Thus, gut microbial shifts do not appear to track closely with the endometriosis lesion stage, though they may correlate with symptom severity (e.g., gastrointestinal complaints) in some patients. [26]
Peritoneal Fluid Microbiome
The peritoneal cavity, once thought to be sterile, can contain a microbiome (or bacterial DNA) introduced via retrograde menstruation or translocation from gut. [27] In endometriosis, refluxed menstrual debris and an inflammatory environment might encourage microbial presence. Several studies have profiled the peritoneal fluid (PF) microbiota in endometriosis patients versus controls undergoing laparoscopy. A consistent observation is that endometriosis PF contains higher levels of certain Gram-positive and Gram-negative bacteria that are uncommon in the PF of controls. Lee et al. (2021) performed shotgun metagenomics on PF from 45 women with stage III–IV endometriosis and 45 controls (with other benign gynecologic conditions). They found a significant increase in the genera Acinetobacter, Pseudomonas, Streptococcus, and Enhydrobacter in endometriosis PF, accompanied by a significant decrease in Propionibacterium (Cutibacterium), Actinomyces, and Rothia compared to controls.[28]
Notably, Propionibacterium, Actinomyces, and Rothia are commensals typically originating from skin or oral flora; their higher presence in control PF could reflect innocuous contamination during surgery that is somehow less prevalent in endometriosis PF, whereas endometriosis PF is enriched in bacteria that may actively proliferate or persist in the peritoneal environment. Wei et al. (2020) similarly reported that Pseudomonas was significantly enriched in endometriosis patients’ peritoneal fluid. They also detected increased Sphingobium (a Gram-negative environmental genus) in endometriosis PF. Wang et al. (2018), using 16S sequencing, found that PF from endometriosis and infertility patients had broadly similar community composition, dominated by Proteobacteria and Firmicutes. This suggests that at higher taxonomic levels, PF microbiota might not differ greatly, but at finer resolution endometriosis PF harbors certain taxa at elevated or reduced abundance.
Importantly, multiple studies converge on Pseudomonas and Streptococcus as PF-enriched genera in endometriosis. These genera include species known to tolerate or thrive in an inflammatory, protein-rich fluid. Acinetobacter (from the Moraxellaceae family) is another frequently found taxon (it corresponds to the Moraxellaceae increase noted in endometrial samples by Khan et al. 2016, and to Acinetobacter in Wei’s endometrial samples. Ruminococcus (a gut-associated genus) has been proposed as a potential biomarker inversely related to endometriosis in some analyses, possibly reflecting its depletion in stool and maybe in PF. One systematic review concluded endometriosis is associated with “an increased abundance of pathogens in peritoneal fluid and a depletion of protective microbes in feces,” citing Pseudomonas (PF) and Ruminococcus (gut) as candidate markers.[29]
In Table 2, we list key genera identified in peritoneal fluid that differ between endometriosis patients and controls.
Table 2. Peritoneal Fluid Microbiome Differences (Endometriosis vs. Controls)
| Genus | Trend in Endometriosis PF | Study/Comments |
|---|---|---|
| Pseudomonas | ↑ Increased | Higher in endo PF in multiple studies [30] |
| Acinetobacter | ↑ Increased | Enriched in endo PF (genus in Moraxellaceae) [31] |
| Streptococcus | ↑ Increased | Higher in endo PF vs control [32] |
| Enhydrobacter | ↑ Increased | Higher in endo PF (genus of Alcaligenaceae) [33] |
| Sphingobium | ↑ Increased | Enriched in endo PF (Wei et al. 2020) [34] |
| Flavobacterium | ↑ (reported) | Found higher in deep endo PF in one study [35][x] |
| Cutibacterium (Propionibacterium) | ↓ Decreased | Lower in endo PF; more abundant in controls [36] |
| Actinomyces | ↓ Decreased | Lower in endo PF (common oral commensal) [37] |
| Rothia | ↓ Decreased | Lower in endo PF [38] |
(PF = peritoneal fluid; ↑ = higher in endo; ↓ = lower in endo, relative to controls)
Major Implications of the Peritoneal Fluid Microbiome Findings
The enrichment of Pseudomonas and Streptococcus in PF suggests a more pro-inflammatory bacterial milieu in endometriosis, as these genera include species that can incite or survive inflammation. Conversely, the relative lack of skin/oral commensals in endo PF might indicate that a healthy peritoneum contains only transient innocuous microbes, whereas an endometriosis-affected peritoneum selectively supports certain bacteria while perhaps clearing others less effectively. It has been hypothesized that retrograde menstruation introduces both endometrial tissue and vaginal microbes (including Gram-negative endotoxin-producing bacteria) into the peritoneal cavity, contributing to lesion development. Further, the concentration of endotoxin lipopolysaccharides (LPS) in peritoneal fluid is significantly higher in women with endometriosis than in controls, as demonstrated by a four- to six-fold increase in menstrual and peritoneal fluid LPS levels in endometriosis patients. [39]
This supports the “bacterial contamination hypothesis,” where E. coli from the gut or vaginal bacteria via menstrual blood introduce LPS into the pelvis, activating Toll-like receptor 4 (TLR4) and downstream inflammation. In summary, the peritoneal cavity in endometriosis appears to have a microbiome skewed toward pro-inflammatory organisms and bacterial products that may perpetuate pelvic inflammation and lesion growth. [40]
Endometrial Microbiome (Eutopic Endometrium)
The uterine cavity (endometrium) has a low-biomass microbiome even in healthy individuals, often dominated by Lactobacillus or a mix of Lactobacillus with other taxa (e.g., Gardnerella, Streptococcus). In endometriosis patients, evidence of altered endometrial microbiota has emerged. Wei et al. (2020) found the eutopic endometrial samples of endometriosis patients were enriched with Sphingobium, Pseudomonas, Delftia, and Acinetobacter compared to controls. [41] These genera are not typical of the healthy endometrium; Sphingobium and Delftia are environmental Gram-negatives (often found in water or lab reagents), but their significant increase in patients suggests they could be true constituents or reflect higher bacterial influx in endo. Pseudomonas and Acinetobacter enrichment aligns with the PF findings above, indicating these organisms might ascend or localize in the uterus as well.
Khan et al. (2016) also examined eutopic endometrial tissue: they reported that in endometriosis, there was a higher presence of Streptococcaceae and Moraxellaceae (the family containing Acinetobacter) in the endometrium compared to controls. [42] This matches Wei’s observation of Streptococcus and Acinetobacter genera increase, but at the family level. Additionally, Wei noted that Escherichia/Shigella (Enterobacteriaceae) were more frequently dominant in the gut of stage III–IV endo patients and other studies have specifically found Escherichia coli to be enriched in endometrial tissue and menstrual blood of endometriosis patients. [43] A study by Chen et al. (2020) also identified E. coli as significantly more abundant in the endometrium of women with endometriosis than in controls. [44] This dovetails with clinical observations that E. coli from refluxed menstrual blood might implant in peritoneal sites, and experimental work showing E. coli LPS can promote endometriosis lesion growth via TLR4 activation.
On the other hand, Hernandes et al. (2020) did not find obvious pathogenic overgrowth in the endometrium of deep endometriosis patients; Lactobacillus was prevalent in both endometriosis and control endometrial samples, with Gardnerella and Prevotella present at lower relative abundance in endometriosis patients. This suggests that perhaps some endometriosis patients maintain a “normal” uterine microbiome similar to healthy women, whereas others show an influx of unusual bacteria (Pseudomonas, Acinetobacter, E. coli, etc.). Differences in study populations or disease subtypes (e.g., ovarian vs. deep endo) could account for these discrepancies. [45]
Advanced disease and Endometrial microbiome
Preliminary data indicate that advanced endometriosis might involve more pronounced endometrial dysbiosis. In Wei’s cohort, stage III–IV patients had a complete absence of Atopobium in the cervix/vagina and higher Gardnerella, Ureaplasma, and Gram-negatives in the cervix which likely extends to the uterus. [46] Another study noted Enterococcus was enriched in endometriosis patients’ cervical mucus but paradoxically depleted in their endometrium – indicating site-specific differences even within the reproductive tract. [47]
In summary, the eutopic endometrium in endometriosis often shows a shift away from a Lactobacillus-dominant community. Consistently increased taxa (vs. controls) include Streptococcus, Enterobacteriaceae (e.g. E. coli), Staphylococcaceae (perhaps via Cutibacterium or Staphylococcus), and Moraxellaceae (Acinetobacter).[48]Escherichia coli in particular has been repeatedly implicated, with one study demonstrating its presence in endometrial tissues and menstrual blood of endo patients but not controls.[49] Decreased taxa in endometriosis endometrium (relative to control endometrium) have been less emphasized, but the reduction of Gardnerella and Prevotella in one study suggests that not all disruptions mirror a classic BV-type overgrowth; some controls (often undergoing surgery for other issues) may have had subclinical endometritis that endometriosis patients lacked.[50] Overall, an endometrial microbial “signature” of endometriosis might be an overrepresentation of facultative anaerobic bacteria that are often associated with inflammation.
Ectopic Lesion Microbiome (Endometriotic Lesions)
The microbiome of ectopic endometriosis lesions (e.g., ovarian endometriomas, deep infiltrating nodules) is challenging to study due to low biomass and potential contamination. However, recent research has shed light on bacteria present within lesions. A groundbreaking 2023 study by Muraoka et al. found Fusobacterium (a Gram-negative anaerobe) in endometriosis lesions and eutopic endometrium of patients, but not in samples from women without endometriosis. [51]Fusobacterium was hypothesized to translocate from the gut or vagina into endometrial tissue. Strikingly, in a mouse model, infection with Fusobacterium facilitated the development of endometriosis lesions, and antibiotic treatment targeting Fusobacterium reduced lesion size. [52] This provides strong evidence of a causal link between at least one bacterium and lesion pathogenesis. Fusobacterium is known for causing periodontal disease and intestinal disorders, so its presence in endometriotic tissue connects to the idea of a microbiome-immune crosstalk driving lesions.
Aside from Fusobacterium, other bacteria have been isolated from lesions. Hernandes et al. (2020) sequenced deep endometriotic lesions and detected DNA of Lactobacillus (dominant genus), as well as Gardnerella, Enterococcus, Pseudomonas, Alishewanella, Ureaplasma, and Aerococcus in lesion samples. [53] The dominance of Lactobacillus in lesions was surprising and might indicate that many detected bacteria were contaminants or transient. Nonetheless, Enterococcus and Pseudomonas (found in lesions) are of interest since they also appear in the vaginal and endometrial profiles of endo patients suggesting possible colonization of lesions by these organisms. [54] Khan et al. (2016) cultured fluid from ovarian endometrioma cysts and identified bacteria as well, although their study focused more on presence/absence than taxonomy. Common vaginal bacteria like Streptococcus agalactiae (Group B strep) have been isolated from ovarian endometriomas in some reports hinting that lesions, especially in ovary, may act as localized infection sites. [55]
It is important to note that healthy controls do not have “lesions” to compare, so lesion microbiome data is usually compared to either the patient’s own eutopic endometrium or to no-microbe controls. Therefore, one can only say which microbes are found in lesions and speculate if they originate from an overgrowth relative to normal tissue. Fusobacterium, for example, is not found in normal endometrium of control women, so its presence in lesions is a notable aberration.[56]
In summary, ectopic endometriosis lesions can harbor bacteria, with Fusobacterium being a prime candidate for a consistently present pathogen in lesions across patients (especially in moderate-severe cases).[57] Other lesion-associated microbes reported include Lactobacillus, Gardnerella, Enterococcus, Streptococcus, Pseudomonas, etc., but these may vary by individual and lesion location. The potential clinical implication is that antibiotic therapies (e.g., against Fusobacterium) might reduce lesion progression, as suggested by the mouse model. Lesion microbiome research is still emerging, but it highlights that what was once thought to be sterile endometriotic tissue may, in fact, host specific microbial communities that contribute to the inflammatory microenvironment.
Oral and Systemic Microbiomes in Endometriosis
Some surprising connections between oral health and endometriosis have been noted. Periodontal disease is more common in women with endometriosis, hinting at a shared inflammatory or microbiome link. A recent prospective study (Hicks et al. 2025) directly profiled the oral microbiota (saliva) of endometriosis patients vs. controls. They found a significant overall difference in composition (p = 0.003) and identified Fusobacterium as a key taxon enriched in the oral samples of patients with moderate/severe endometriosis.[58]Fusobacterium (notably F. nucleatum) is a periodontal pathogen; its higher abundance in endo patients’ mouths correlates with the above-mentioned detection of Fusobacterium in their lesions. This suggests a possible oral-genital microbial axis: chronic gum infection with Fusobacterium might seed distant sites or drive systemic inflammation. Aside from Fusobacterium, Hicks et al. reported other oral differences by disease severity: for instance, Cardiobacterium (another oral anaerobe) was enriched in minimal/mild endometriosis, whereas Fusobacterium was enriched in moderate/severe cases. [59]
These differences are visualized in Figure 1, which summarizes taxa distinguishing mild vs. severe endometriosis across oral, stool, and vaginal sites.
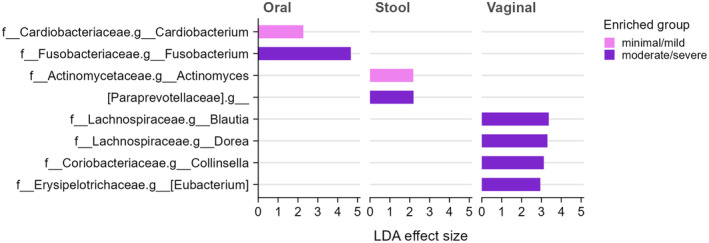
Figure 1. Differential microbial taxa between minimal/mild vs. moderate/severe endometriosis, stratified by body site (oral, stool, vaginal). Pink bars indicate taxa enriched in minimal/mild stage endometriosis; purple bars indicate enrichment in moderate/severe stage. For example, in the oral microbiome, Cardiobacterium is higher in mild cases, while Fusobacterium is higher in moderate/severe cases. In stool, Actinomyces is enriched in mild endo, whereas a family Paraprevotellaceae (an anaerobe group) is enriched in severe endopmc.ncbi.nlm.nih.gov. In the vaginal microbiome, several genera (Blautia, Dorea, Collinsella, Eubacterium) show higher abundance in moderate/severe endometriosis (purple)pmc.ncbi.nlm.nih.gov. No taxa were enriched in mild cases for the vaginal site in this analysis, reflecting that differences were more apparent in severe disease.[60]
Beyond Fusobacterium, the oral microbiome of endometriosis patients showed that some commensal genera were less abundant compared to controls. Although not the main focus of most studies, one can infer from the PF data that genera like Rothia, Actinomyces, and Propionibacterium – which decreased in endometriosis peritoneal fluid – might also be altered in the mouth.[61] In fact, Actinomyces was found to be enriched in the gut of mild endo patients (perhaps from swallowed oral flora) but not in severe cases.[62] The interaction between oral and gut microbiomes (via ingestion of oral bacteria) could mean oral dysbiosis influences gut and vice versa.
Crucially, the finding of periodontal pathogens in endometriosis (oral cavity and lesions) underscores the importance of systemic microbial spread. If oral bacteria like Fusobacterium can translocate to the uterus or peritoneum (via bloodstream or other routes), targeting periodontal disease might conceivably reduce endometriosis risk or severity. Hicks et al. conclude that the oral, gut, and vaginal microbiota all show distinct signatures in endometriosis, and propose a diagnostic microbiome-based swab might be developed.[63]
Systemic (blood) microbiome and immune response
While blood is normally sterile, researchers have looked for microbial DNA in circulation as a sign of a “systemic microbiome.” Direct 16S sequencing of blood in endometriosis is rare (due to low microbial load), but indirect evidence of microbial translocation exists. One such evidence is elevated endotoxin levels in circulation or pelvic fluids of endometriosis patients. As noted earlier, menstrual fluid and peritoneal fluid in endometriosis contain significantly higher LPS levels (detected by Limulus assay) than those in controls. [64] This LPS can enter the bloodstream, especially if endometriosis is associated with increased intestinal permeability. Cadmium exposure in animal models, for instance, increased gut permeability and systemic inflammationwhich facilitates endotoxin leakage. In endometriosis patients, one study found evidence of LPS in the pelvic cavity activating TLR4 on macrophages, leading to inflammation and lesion growth. [65]
Another systemic aspect is the immune response to microbial components. Endometriosis lesions often have activated macrophages and neutrophils; these immune cells can respond to microbial molecules like LPS or peptidoglycans. The presence of gram-negative bacteria such as Escherichia coli in endometriosis can chronically stimulate TLR4-mediated pathways.[66] Systemically, endometriosis patients have been reported to have a heightened inflammatory cytokine profile (e.g., elevated IL-6, TNF-α), which could partly be driven by microbial endotoxins crossing into circulation.
Taken together, systemic microbial signatures in endometriosis are subtle but plausible. They likely manifest not as a detectable blood microbiome community per se, but as elevated microbial products (LPS, bacterial metabolites) and corresponding immune activation in blood. For example, a recent study linked an “endometriosis-specific vaginal microbiota” with changes in urinary and serum metabolites (including possibly Ni or other metal ion levels, see below). [67] This hints that microbiome shifts can have systemic metabolic effects measurable in blood/urine. Ongoing research is examining bacterial extracellular vesicles in blood and PF, which might carry microbial signals throughout the body.[68] In summary, the systemic implications of the endometriosis microbiome include chronic exposure to bacterial endotoxins (LPS) and metabolic byproducts that sustain inflammation beyond the pelvis.
Conclusion
Over the last 20 years, research has increasingly implicated microbiome dysbiosis in the pathophysiology of endometriosis. Although patients and controls share many of the same microbes, significant differences in relative abundance have been documented in multiple compartments (Table 3). Early-stage endometriosis may have relatively minor microbiome perturbations, but advanced disease and associated inflammation correlate with more pronounced shifts: for instance, a less Lactobacillus-dominated vagina, a gut microbiome with subtle increases in pro-inflammatory genera, an infusion of bacteria into the normally low-biomass endometrium, and a peritoneal fluid microbiome skewed toward facultative anaerobes and opportunists. A particularly robust finding is the association of Fusobacterium with endometriosis, spanning oral to pelvic sites, suggesting a possible causal microbe that bridges distant body sites.
Table 3. Summary of Microbiome Changes in Endometriosis vs. Healthy Controls by Compartment
| Compartment | Endometriosis vs. Control | Consistently Increased Taxa | Consistently Decreased Taxa |
|---|---|---|---|
| Vagina | Often Lactobacillus-dominant in both, but endo shows slight dysbiosis in some studies [69] | Gardnerella, Prevotella, Aerococcus, Anaerococcus, Veillonella, Clostridium (in various studies; more anaerobes in symptomatic or advanced cases). [70] | Lactobacillus (sometimes ↓ modestly), Atopobiumpmc.ncbi.nlm.nih.gov, Megasphaera, Shuttleworthia (in endo + pain), Gardnerella (↓ in one study).[71] |
| Gut (Stool) | Subtle differences; overall microbiota often similarpmc.ncbi.nlm.nih.gov. Some specific genus shifts reported. | Lachnospira, Oscillospira, [72]Coprococcus, Bacteroides (some studies) – generally Firmicutes and certain Bacteroidetes higher in a few reports. Also Phascolarctobacterium noted higher in endo stool and PF.[73] | Sneathia, Barnesiella, Gardnerella[74] (significantly ↓ in one study); Paraprevotella, Ruminococcaceae (trends toward lower in endo, correlating with inflammation. [75] |
| Peritoneal Fluid | Not sterile; endo PF has higher bacterial load or DNA (including LPS). Distinct taxa profile in endo vs. control. [76] | Pseudomonas, Acinetobacter, Streptococcus, Enhydrobacter, Sphingobium, Flavobacterium (one study) – these are elevated in endo PF, indicating more Gram-negative and aerobic/facultative bacteria. [77] | Propionibacterium (Cutibacterium), Actinomyces, Rothia – significantly lower in endo PF (these skin/oral commensals found more in control PF). [78] |
| Eutopic Endometrium (Uterus) | Low biomass in health; endo often shows presence of atypical bacteria. | Streptococcus (↑ Streptococcaceae),Escherichia coli, Enterococcus, Acinetobacter (Moraxellaceae)pmc.ncbi.nlm.nih.gov, Gardnerella (some reports), Fusobacterium (found in endo tissue, not in controls). [79][80] | Lactobacillus (when comparing to controls, endo often has less lacto dominance). Prevotella and Gardnerella were lower in one deep endo study, suggesting not all endo uteri have BV-like overgrowth. [81] |
| Ectopic Lesions | N/A in controls (no lesions). Lesions can harbor bacteria in endo patients. | Fusobacterium(frequently found in lesions), Lactobacillus (found in lesions in some sequencing studies, Pseudomonas, Enterococcus, Streptococcus, Ureaplasma (detected in some lesion samples).[82] | (No direct control comparison; however, these taxa are not present in normal peritoneum, so “decreased” not applicable. Healthy peritoneum essentially has none of these as resident microbes.) |
| Oral Cavity | Endo patients’ oral microbiome composition differs (p=0.003). | Fusobacterium (notably in moderate/severe endo), Dialister, Megasphaera (periodontal anaerobes, possibly higher – needs confirmation), Prevotella (periodontal species might increase). Also Campylobacter and Cardiobacterium in some mild cases. [83][84] | Streptococcus, Actinomyces, Rothia, Neisseria (these normal oral commensals might be relatively lower in endometriosis – inferred from PF data where they were decreased (possibly reflecting oral dysbiosis).[85] |
| Systemic (Blood) | Higher endotoxin (LPS) levels in endo vs control. No distinct “blood microbiome” community identified (blood is normally sterile). [86] | N/A (blood should not have taxa). Indirectly, endotoxins from E. coli or gut bacteria are elevated. Also bacterial metabolites (perhaps higher TMAO or others if gut dysbiosis).[87] | N/A. Possibly lower levels of short-chain fatty acids systemically if gut butyrate-producers are reduced (e.g. less Ruminococcus could mean less circulating butyrate – an anti-inflammatory). |
Finally, an integrative approach considering both microbes and metal microenvironments could yield novel insights into endometriosis. Therapies aimed at modulating the microbiome – probiotics, targeted antibiotics (e.g., against Fusobacterium, or even fecal microbiota transplantation – alongside dietary and environmental interventions to reduce toxic metal exposure, might together alleviate some of the inflammatory burden of endometriosis. The consistent themes of inflammation, dysbiosis, and metallomics reinforce that endometriosis is not solely a hormonal disease, but a systemic condition with infectious and environmental components. As research continues, the hope is that microbiome signatures (such as a combination of vaginal Escherichia/Shigella and Enterococcus abundance, or oral Fusobacterium levels) could be developed into non-invasive biomarkers for earlier endometriosis diagnosis, and that correcting the dysbiosis and underlying metal imbalances might become a part of comprehensive endometriosis care.
Endometriosis involves ectopic endometrial tissue causing pain and infertility. Validated and Promising Interventions include Hyperbaric Oxygen Therapy (HBOT), Low Nickel Diet, and Metronidazole therapy.
Facultative anaerobes are microorganisms—primarily bacteria—that possess the metabolic flexibility to grow in both the presence and absence of oxygen.
Lipopolysaccharide (LPS), a potent endotoxin present in the outer membrane of Gram-negative bacteria that causes chronic immune responses associated with inflammation.
Facultative anaerobes are microorganisms—primarily bacteria—that possess the metabolic flexibility to grow in both the presence and absence of oxygen.
Facultative anaerobes are microorganisms—primarily bacteria—that possess the metabolic flexibility to grow in both the presence and absence of oxygen.
Facultative anaerobes are microorganisms—primarily bacteria—that possess the metabolic flexibility to grow in both the presence and absence of oxygen.
TMAO is a metabolite formed when gut bacteria convert dietary nutrients like choline and L-carnitine into trimethylamine (TMA), which is then oxidized in the liver to TMAO. This compound is linked to cardiovascular disease, as it promotes atherosclerosis, thrombosis, and inflammation, highlighting the crucial role of gut microbiota in influencing heart health.
References
- The bidirectional relationship between endometriosis and microbiome.. Uzuner C, Mak J, El-Assaad F, Condous G.. (Frontiers in Endocrinology. 2023.)
- Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms.. Ser HL, Au Yong SJ, Shafiee MN, Mokhtar NM, Ali RAR.. (Microorganisms. 2023.)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.. Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.. (Biomed Res Int. 2023)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.. Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.. (Biomed Res Int. 2023)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.. Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.. (Biomed Res Int. 2023)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Gut microbiome in endometriosis: a cohort study on 1000 individuals.. Pérez-Prieto I, Vargas E, Salas-Espejo E, Lüll K, Canha-Gouveia A, Pérez LA, Fontes J, Salumets A, Andreson R, Aasmets O. (Erratum in: BMC Med. 2024.)
- Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms.. Ser HL, Au Yong SJ, Shafiee MN, Mokhtar NM, Ali RAR.. (Microorganisms. 2023.)
- The bidirectional relationship between endometriosis and microbiome.. Uzuner C, Mak J, El-Assaad F, Condous G.. (Frontiers in Endocrinology. 2023.)
- Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms.. Ser HL, Au Yong SJ, Shafiee MN, Mokhtar NM, Ali RAR.. (Microorganisms. 2023.)
- Gut microbiome in endometriosis: a cohort study on 1000 individuals.. Pérez-Prieto I, Vargas E, Salas-Espejo E, Lüll K, Canha-Gouveia A, Pérez LA, Fontes J, Salumets A, Andreson R, Aasmets O. (Erratum in: BMC Med. 2024.)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Gut microbiome in endometriosis: a cohort study on 1000 individuals.. Pérez-Prieto I, Vargas E, Salas-Espejo E, Lüll K, Canha-Gouveia A, Pérez LA, Fontes J, Salumets A, Andreson R, Aasmets O. (Erratum in: BMC Med. 2024.)
- Bacterial contamination hypothesis: a new concept in endometriosis.. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.. (Reprod Med Biol. 2018)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.. Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.. (Biomed Res Int. 2023)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Peritoneal fluid microbiota profile of patients with deep endometriosis.. Malvezzi H, Cestari BA, Mendes H, Hernandes C, Podgaec S.. (Microb Pathog. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Bacterial contamination hypothesis: a new concept in endometriosis.. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.. (Reprod Med Biol. 2018)
- Bacterial contamination hypothesis: a new concept in endometriosis.. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.. (Reprod Med Biol. 2018)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.. Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.. (Biomed Res Int. 2023)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.. Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.. (Biomed Res Int. 2023)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Bacterial contamination hypothesis: a new concept in endometriosis.. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.. (Reprod Med Biol. 2018)
- Cadmium exposure induces changes in gut microbial composition and metabolic function in long-tailed dwarf hamsters, Cricetulus longicaudatus.. Tao M, Cao K, Pu X, Hou Y, He L, Liu W, Ren Y, Yang X.. (Ecol Evol. 2024)
- Bacterial contamination hypothesis: a new concept in endometriosis.. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.. (Reprod Med Biol. 2018)
- Endometriosis specific vaginal microbiota links to urine and serum N-glycome.. MacSharry, J., Kovács, Z., Xie, Y. et al.. (Sci Rep 14, 25372 (2024).)
- Altered Composition of Microbiota in Women with Ovarian Endometrioma: Microbiome Analyses of Extracellular Vesicles in the Peritoneal Fluid.. Lee SR, Lee JC, Kim SH, Oh YS, Chae HD, Seo H, Kang CS, Shin TS.. (Int J Mol Sci. 2021)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review.. Ser HL, Au Yong SJ, Shafiee MN, Mokhtar NM, Ali RAR.. (Microorganisms. 2023.)
- Bacterial contamination hypothesis: a new concept in endometriosis.. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.. (Reprod Med Biol. 2018)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.. Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.. (BJOG. 2025)
- Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.. Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.. (Biomed Res Int. 2023)
- The microbiome and endometriosis.. Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.. (Reprod Fertil. 2022)
- Bacterial contamination hypothesis: a new concept in endometriosis.. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.. (Reprod Med Biol. 2018)
- Bacterial contamination hypothesis: a new concept in endometriosis.. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.. (Reprod Med Biol. 2018)
Uzuner C, Mak J, El-Assaad F, Condous G.
The bidirectional relationship between endometriosis and microbiome.Frontiers in Endocrinology. 2023.
Ser HL, Au Yong SJ, Shafiee MN, Mokhtar NM, Ali RAR.
Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms.Microorganisms. 2023.
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.
Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.Biomed Res Int. 2023
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.
Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.Biomed Res Int. 2023
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.
Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.Biomed Res Int. 2023
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Pérez-Prieto I, Vargas E, Salas-Espejo E, Lüll K, Canha-Gouveia A, Pérez LA, Fontes J, Salumets A, Andreson R, Aasmets O
Gut microbiome in endometriosis: a cohort study on 1000 individuals.Erratum in: BMC Med. 2024.
Ser HL, Au Yong SJ, Shafiee MN, Mokhtar NM, Ali RAR.
Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms.Microorganisms. 2023.
Uzuner C, Mak J, El-Assaad F, Condous G.
The bidirectional relationship between endometriosis and microbiome.Frontiers in Endocrinology. 2023.
Ser HL, Au Yong SJ, Shafiee MN, Mokhtar NM, Ali RAR.
Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review. Microorganisms.Microorganisms. 2023.
Pérez-Prieto I, Vargas E, Salas-Espejo E, Lüll K, Canha-Gouveia A, Pérez LA, Fontes J, Salumets A, Andreson R, Aasmets O
Gut microbiome in endometriosis: a cohort study on 1000 individuals.Erratum in: BMC Med. 2024.
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Pérez-Prieto I, Vargas E, Salas-Espejo E, Lüll K, Canha-Gouveia A, Pérez LA, Fontes J, Salumets A, Andreson R, Aasmets O
Gut microbiome in endometriosis: a cohort study on 1000 individuals.Erratum in: BMC Med. 2024.
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.
Bacterial contamination hypothesis: a new concept in endometriosis.Reprod Med Biol. 2018
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.
Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.Biomed Res Int. 2023
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Malvezzi H, Cestari BA, Mendes H, Hernandes C, Podgaec S.
Peritoneal fluid microbiota profile of patients with deep endometriosis.Microb Pathog. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.
Bacterial contamination hypothesis: a new concept in endometriosis.Reprod Med Biol. 2018
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.
Bacterial contamination hypothesis: a new concept in endometriosis.Reprod Med Biol. 2018
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.
Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.Biomed Res Int. 2023
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.
Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.Biomed Res Int. 2023
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.
Bacterial contamination hypothesis: a new concept in endometriosis.Reprod Med Biol. 2018
Tao M, Cao K, Pu X, Hou Y, He L, Liu W, Ren Y, Yang X.
Cadmium exposure induces changes in gut microbial composition and metabolic function in long-tailed dwarf hamsters, Cricetulus longicaudatus.Ecol Evol. 2024
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.
Bacterial contamination hypothesis: a new concept in endometriosis.Reprod Med Biol. 2018
MacSharry, J., Kovács, Z., Xie, Y. et al.
Endometriosis specific vaginal microbiota links to urine and serum N-glycome.Sci Rep 14, 25372 (2024).
Lee SR, Lee JC, Kim SH, Oh YS, Chae HD, Seo H, Kang CS, Shin TS.
Altered Composition of Microbiota in Women with Ovarian Endometrioma: Microbiome Analyses of Extracellular Vesicles in the Peritoneal Fluid.Int J Mol Sci. 2021
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Ser HL, Au Yong SJ, Shafiee MN, Mokhtar NM, Ali RAR.
Current Updates on the Role of Microbiome in Endometriosis: A Narrative Review.Microorganisms. 2023.
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.
Bacterial contamination hypothesis: a new concept in endometriosis.Reprod Med Biol. 2018
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Hicks C, Leonardi M, Chua XY, Mari-Breedt L, Espada M, El-Omar EM, Condous G, El-Assaad F.
Oral, Vaginal, and Stool Microbial Signatures in Patients With Endometriosis as Potential Diagnostic Non-Invasive Biomarkers: A Prospective Cohort Study.BJOG. 2025
Colonetti T, Saggioratto MC, Grande AJ, Colonetti L, Junior JCD, Ceretta LB, Roever L, Silva FR, da Rosa MI.
Gut and Vaginal Microbiota in the Endometriosis: Systematic Review and Meta-Analysis.Biomed Res Int. 2023
Miyashira CH, Oliveira FR, Andres MP, Gingold JA, Abrão MS.
The microbiome and endometriosis.Reprod Fertil. 2022
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.
Bacterial contamination hypothesis: a new concept in endometriosis.Reprod Med Biol. 2018
Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, Kitawaki J.
Bacterial contamination hypothesis: a new concept in endometriosis.Reprod Med Biol. 2018
